Editorial Policies
The International Journal of Medicine and Health Innovations Perspectives (IJOMAHIP) is an open-access, peer-reviewed journal dedicated to advancing knowledge in the fields of Medicine and Health Sciences fostering innovation, facilitating global knowledge exchange, and supporting collaborative efforts among researchers, clinicians, educators, and healthcare practitioners worldwide.
Table of Contents
Overview
IJOMAHIP adheres to the standards set by the Committee on Publication Ethics (COPE) and supports the World Association of Medical Editors (WAME) Policy Statement on Geopolitical Intrusion on Editorial Decisions. IJOMAHIP also follows the International Committee of Medical Journal Editors (ICMJE) Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals.
By submitting a manuscript to IJOMAHIP, all authors confirm that they have read and agree to the journal’s content and policies.
IJOMAHIP maintains neutrality regarding jurisdictional claims in published maps and institutional affiliations.
Open Access
All articles published in IJOMAHIP are immediately available online for free and without any subscription fees or registration requirements upon publication. As authors of these articles, you retain copyright ownership and grant third parties the perpetual right to use, reproduce, or distribute your article in accordance with the specified license terms.
IJOMAHIIP guarantees worldwide accessibility to published research, facilitating the unhindered dissemination of knowledge and promoting academic collaboration across borders.
Professional Conduct
IJOMAHIP believes that mutual respect is the foundation for trust and high-quality publishing. We expect professional and respectful behavior from our staff in all interactions with authors, reviewers, and readers. Similarly, we expect the same standards of behavior from the academic community and the public when interacting with our staff. Aggressive behavior, harassment, bullying, or discrimination directed at IJOMAHIP staff will not be tolerated. We reserve the right to report serious violations to employers or local authorities and may refuse to interact with individuals who repeatedly or seriously violate this policy.
Ethical Guidelines for Research Involving Human Participants
Authors are required to follow ethical research practices and adhere to relevant regulations.
Ethics approval
- Research involving human participants, material, or data must comply with the Declaration of Helsinki and be approved by an appropriate ethics committee.
- All manuscripts reporting such research must include a statement detailing this approval, including the ethics committee’s name and reference number (if applicable).
- If a study was exempt from ethics approval, this must also be stated, including the name of the granting committee.
- Manuscripts may be rejected if the Chief Editor deems the research unethical. The Chief Editor may contact the ethics committee for more information in rare cases.
Retrospective ethics approval
- Retrospective ethics approval is usually not possible if a study commenced without prior ethics committee approval.
- The Editor has discretion over whether to proceed with peer review in such cases.
New clinical tools and procedures
- Authors reporting the use of new procedures or tools in a clinical setting must justify why the new approach was more appropriate than standard practice to meet the patient’s clinical need. This justification is not needed if the procedure is already approved for clinical use at the authors’ institution.
- Ethics committee approval and informed patient consent are required for any experimental use of novel procedures/tools where a clear clinical advantage was not apparent before treatment.
Consent to participate
- Informed consent to participate in the study must be obtained from all human participants (or their parent/legal guardian for those under 16). A statement confirming this should be included in the manuscript.
- Manuscripts involving vulnerable groups, potential coercion (e.g., prisoners), or cases where consent may not have been fully informed will be considered at the Chief Editor’s discretion and may be referred to an internal editorial oversight group.
- Consent must be obtained for all forms of personally identifiable data (biomedical, clinical, biometric, etc.).
- For human transplantation studies, authors must declare that no organs/tissues were obtained from prisoners and must name the institutions/clinics/departments through which organs/tissues were obtained.
- Documentary evidence of consent must be provided upon request.
Ethical Guidelines for Research Involving Human Embryos, Gametes, and Stem Cells
Manuscripts reporting experiments involving human embryos and gametes, human embryonic stem cells and related materials, and clinical applications of stem cells must confirm that all experiments were performed according to relevant guidelines and regulations.
- The manuscript must include an ethics statement identifying the institutional and/or national research ethics committee (including the committee’s name) that approved the experiments and describing relevant details.
- Authors should confirm that informed consent was obtained from all recipients and/or donors of cells or tissues, where necessary, and describe the conditions of donation of materials for research, such as human embryos or gametes.
- The Chief Editor may request copies of approval and redacted consent documents.
- Authors are encouraged to follow the principles outlined in the 2016 ISSCR Guidelines for Stem Cell Research and Clinical Translation.
When deciding whether to publish papers describing modifications of the human germline, the Chief Editor will consider safety, compliance with regulations, and the societal debate on the implications of such modifications for future generations.
The final decision to publish is the responsibility of the Chief Editor.
Guidelines for Sex and Gender Equity in Research (SAGER)
We encourage authors to follow the Sex and Gender Equity in Research (SAGER) guidelines and include sex and gender considerations where relevant. Authors should use the terms “sex” (biological attribute) and “gender” (shaped by social and cultural circumstances) carefully to avoid confusion. Article titles and abstracts should clearly indicate the sex(es) to which the study applies.
Authors should also:
- Describe in the background whether sex and/or gender differences may be expected.
- Report how sex and/or gender were accounted for in the design of the study.
- Provide disaggregated data by sex and/or gender, where appropriate.
- Discuss respective results.
- If a sex and/or gender analysis was not conducted, provide the rationale in the Discussion.
We suggest that our authors consult the full guidelines before submission. These guidelines apply to studies involving humans, vertebrate animals, and cell lines.
Definitions (Adapted from Office of Research in Women’s Health, NIH):
- Sex: Refers to biological differences between females and males, including chromosomes, sex organs, and endogenous hormonal profiles
- Gender: Refers to socially constructed and enacted roles and behaviors that occur in a historical and cultural context and vary across societies and over time
- Applications of the guidelines: These guidelines apply to studies involving humans, vertebrate animal and cell lines.
Research Involving Animals
- A statement detailing compliance with relevant guidelines (e.g., the Animals (Scientific Procedures) Act 1986 in the UK and Directive 2010/63/EU in Europe) and/or ethical approval (including the name of the ethics committee and the reference number where appropriate) must be included in the manuscript.
- If a study has been granted an exemption from requiring ethics approval, this should also be detailed in the manuscript, including the name of the ethics committee that granted the exemption and the reasons for the exemption.
- The Chief Editor will consider animal welfare issues and may reject a manuscript if the research involves protocols inconsistent with commonly accepted norms of animal research. In rare cases, the Chief Editor may contact the ethics committee for further information.
Manuscripts presenting studies that have employed anesthesia or euthanasia methods inconsistent with commonly accepted norms of veterinary best practice (e.g., chloral hydrate, ether, and chloroform) will not be considered. Decisions to not consider manuscripts presenting such anesthesia or euthanasia methods are independent of the approving ethics committee and any previously published work. Authors should consult the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals (2020) for guidance on veterinary best practice for the anesthesia and euthanasia of animals.
For experimental studies involving client-owned animals, authors must also document informed consent from the client or owner and adherence to a high standard (best practice) of veterinary care.
Field studies and other non-experimental research on animals must comply with institutional, national, or international guidelines and, where available, should be approved by an appropriate ethics committee. A statement detailing compliance with relevant guidelines and/or appropriate permissions or licenses must be included in the manuscript. Authors are encouraged to comply with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
The use of animals for research, teaching, and testing is a privilege that carries with it important responsibilities:
- Ensure that good science is conducted
- Meet ethical responsibilities for ensuring that every animal is treated humanely and not subjected to unnecessary pain or distress
- Work within the accepted standards for experimental animal care and use
When animals are used in science or other activities, the primary concern is to minimize discomfort to the animals and ensure that their care and use be in accordance with guidelines. Some core values are embedded in the “Three Rs” policy concept of replacement, reduction, and refinement of animals. The Three Rs stipulate that harm be minimized when animals are being used for scientific pursuits.
Research Involving Plants
Experimental research and field studies on plants (cultivated or wild), including the collection of plant material, must comply with relevant institutional, national, and international guidelines and legislation.
- Manuscripts should include a statement specifying the appropriate permissions and/or licenses for the collection of plant or seed specimens.
- Authors are encouraged to comply with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
To support reproducibility, voucher specimens for all wild plants described in a manuscript must be deposited in a public herbarium or other public collection that provides access to deposited material. Information on the voucher specimen and who identified it must be included in the manuscript.
Research Involving Palaeontological and Geological Material
For research involving paleontological specimens and geological samples, clear provenance information is essential for transparency.
- While it is recognized that precise provenance may be unavailable for older museum collections, and detailed locality information may be excluded if it compromises site security, samples must always be collected and exported responsibly and in accordance with local and national laws.
- Submissions detailing new material should include information regarding obtained permissions and the issuing authority. Authors may be required to provide specific supporting documentation upon request.
- Type, figured, and cited paleontological specimens should be deposited in a recognized museum or collection for free access by other researchers in perpetuity. Sufficient repository information, including assigned unique catalog numbers (where applicable), should be provided for specimen traceability.
- Deposition of 3-D scans of fossil specimens (where appropriate) within a permanent, accessible repository is encouraged to facilitate scientific community study.
IJOMAHIP requires submitted content to adhere to theUnited Nations Educational, Scientific and Cultural Organization (UNESCO) normative instruments for the protection of cultural heritage, and Resolutions, Motions, guidance and other statements of the International Union for the Conservation of Nature (IUCN).
Dual Use Research of Concern
IJOMAHIP recognizes that some manuscripts may contain information that could be misused to pose a significant threat to public health, safety, security, agriculture, animals, or the environment. Publication of such information requires the benefits to the research community, society, or public health to outweigh any potential risks.
- We reserve the right to seek expert advice and may subject manuscripts to peer review specifically to assess dual-use risk.
- If the risk of misuse outweighs potential benefits, publication will be declined. Published content may be corrected, retracted, or removed.
Researchers are expected to:
- Comply with institutional and funder requirements, as well as national regulations.
- Be aware of dual-use concerns related to their work and take steps to minimize misuse, including biosecurity, nuclear, and chemical threats.
- Disclose whether their study is subject to consideration as dual-use research of concern, reporting the authority granting approval and the reference number if applicable.
- Describe appropriate containment procedures (e.g., biosafety) when the study reports material that can be harmful outside the laboratory context.
While we acknowledge the potential risks, we also recognize the widespread view that openness in science helps to alert society to potential threats and to defend against them. We anticipate that only rarely will the risks be perceived as outweighing the benefits of publishing a paper that has otherwise been deemed appropriate for publication.
Research Standards for Complementary and Alternative Medicine
IJOMAHIP is dedicated to evidence-based research and believes that Complementary and Alternative Medicine (CAM) research should adhere to the same rigorous standards and evidence thresholds as conventional medicine research.
We welcome submissions that meet the following clinical research standards:
- Compliance: Clinical research manuscripts must comply with international and national standards, such as the Declaration of Helsinki or relevant governmental regulations (e.g., the UK’s The Medicines for Human Use (Clinical Trials) Regulations).
- Study design: Studies must be adequately controlled (compared to a placebo or conventional medicine), blinded where appropriate, randomized, and have sufficient statistical power to interpret the reported effect confidently and accurately. Comparing a CAM treatment/ technique only to another CAM treatment/ technique is insufficient to test the efficacy of the CAM treatment in question. Studies supplementing a conventional treatment with a CAM technique are only valid if compared to the same conventional treatment supplemented with a placebo.
- Prior evidence: For CAM treatments/ techniques tested on animal models and/or human patients, it is unethical for such work to occur without adequate prior evidence that the treatment/technique shows some therapeutic potential. Manuscripts must include objective, measurable data from previously published peer-reviewed literature adhering to scientific principles (e.g., in vitro or cellular work). Other forms of evidence are not valid, and manuscripts lacking this evidence will not be considered on ethical grounds.
Guidelines for Consent for Publication
The journal adheres to the Committee on Publication Ethics (COPE) guidelines to ensure ethical publishing practices.
For manuscripts that include details, images, or videos relating to an individual person, written informed consent for publication must be obtained from that person (or their parent or legal guardian if under 18).
- The consent must be for publication of their details under the relevant Creative Commons license, ensuring they are freely available on the internet.
- If the person has died, consent must be obtained from their next of kin.
- The manuscript must state that written informed consent for publication was obtained.
Human Research Participant Publication Approval Templates (available in English and other languages/dialects) can be used to obtain consent where identifiable information and/or media are present. The consent form must state that the details/images/videos will be freely available on the internet and may be seen by the general public.
If images are entirely unidentifiable and no details on individuals are reported, consent for publication may not be required. The Chief Editor has the final decision on whether consent is needed.
Further information, including Third Party Rights for dissemination, is available on the IJOMAHIP pages.
Trial Registration Policy
IJOMAHIP supports initiatives to improve clinical trial reporting, including prospective registration in publicly available databases. In line with ICMJE guidelines, IJOMAHIP requires registration of all clinical trials reported in submitted manuscripts.
Definition of clinical trial: IJOMAHIP follows the World Health Organization (WHO) definition of a clinical trial: “any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes”. This includes phase I to IV trials. Health-related interventions are defined as “any intervention used to modify a biomedical or health-related outcome,” and health-related outcomes are “any biomedical or health-related measures obtained in patients or participants”.
Suitable registries: Acceptable registries are those listed on the ICMJE website and any primary registries participating in the WHO International Clinical Trials Registry Platform (ICTRP), including the ISRCTN registry. You cannot register a trial with WHO directly.
Registration information in manuscript: The trial registration number (TRN) and date of registration should be included as the last line of the manuscript abstract.
Publication of study protocols: Publication of study protocols reduces the risk of non-publication of research findings and facilitates methodological discussion, and is encouraged by a number of IJOMAHIP. If the study protocol for a trial has been published, it should be cited in the manuscript.
Retrospective registration: For clinical trials that have not been registered prospectively, IJOMAHIP encourages retrospective registration to ensure complete publication of all results. Many journals published by IJOMAHIP will consider manuscripts describing retrospectively registered studies. The TRN, date of registration, and the words “retrospectively registered” should be included as the last line of the manuscript abstract.
Systematic reviews
IJOMAHIP supports the prospective registration of systematic reviews and encourages authors to register their systematic reviews in a suitable registry (such as PROSPERO). Authors who have registered their systematic review should include the registration number as the last line of the manuscript abstract.
The ICMJE requires registration of clinical trials in a public trials registry at or before the time of first patient enrollment as a condition of consideration for publication. Trials must be registered before any participants are enrolled. Failing to register a clinical trial, or keep the information up to date or submit false/misleading information may lead to the inability to publish results in an ICMJE-associated journal.
Data and Materials Availability Policy
IJOMAHIP is designed to support our commitment to open data sharing. By submitting a manuscript to an IJOMAHIP journal, authors agree to make all materials described in the manuscript, including relevant raw data, freely available to scientists for non-commercial purposes, without compromising participant confidentiality.
IJOMAHIP strongly encourages the availability of all datasets supporting the paper’s conclusions. Where a community norm for data sharing exists, IJOMAHIP mandates data deposition (see specific data types below).
- Authors are encouraged to deposit datasets in publicly available repositories (where available and appropriate) or present them in the main manuscript or supporting files in machine-readable format (e.g., spreadsheets).
- Data deposition is mandatory which the conclusions of the manuscript rely is required
- For a complete list of approved repositories, please visit the Data Repository Guidance page.
Authors needing assistance with data sharing policies, finding suitable repositories, or organizing and sharing research data can access our Author Support portal for guidance.
Mandated data deposition policy
IJOMAHIP requires data deposition in a public access repository for certain data types with community-accepted standards of data deposition and data sharing (e.g., genomic data, nucleic acid, or protein sequences) upon submission.
- Consult the list of mandated data types to ensure that any relevant data are deposited in an appropriate repository and linked from your manuscript.
- This data must be available to editors and reviewers for evaluation during peer review and must be released to the public without restriction upon publication.
Data types with required deposition include:
- Macromolecular structure data (e.g., protein, RNA, DNA structures)
- Crystallographic data
- Nucleic acid and protein sequences
- Gene expression data
- Genomic data
- Mutant and transgenic animal data
- Proteomics data
- Metabolomics data
- Chemical structures
Special considerations
- DNA and RNA Sequences: Deposition of novel DNA and RNA sequence and novel genome assembly data is mandated. We strongly encourage depositions of all DNA and RNA sequences. These sequences and assemblies must be deposited in repositories that are part of the International Nucleotide Sequence Collaboration (INSDC) or those working toward INSDC inclusion. When publishing reference genomes, both the assembly and raw sequence reads must be available. Sequence deposition is required even for short stretches of novel sequence information like epitopes, functional domains, genetic markers, or haplotypes. We encourage supplementing short novel sequences with surrounding sequence information for context. The sequences of all small RNA probes central to the conclusions of the paper must be provided. We highly encourage the deposition of microbial assemblies derived from metagenomics data.
- Genomics and Transcriptomics Datasets: When depositing genomic and transcriptomics datasets, we encourage providing metadata to allow for dataset reproduction. We also encourage including annotations where applicable, especially when presenting data of unsequenced genomes.
- Linked Phenotype and Genotype Data for Human Subjects: This data should be submitted to a publicly accessible repository with appropriate access controls. Any restrictions on data access for sensitive data (e.g., electronic medical records, forensic data, personal data from vulnerable populations) require an explanation of the nature of and reasons for the restrictions, and details of the conditions under which the data can be accessed or reused.
- Gene Expression Data: Data derived from microarray studies must be MIAME compliant (https://fairsharing.org/bsg-s000177/).
Publication of clinical datasets guidelines
When publishing datasets containing clinical data, authors have ethical and legal responsibilities to respect participants’ privacy and protect their identity.
- Ideally, obtain informed consent for dataset publication from participants at the point of recruitment to the study or trial.
- If obtaining consent is impossible, demonstrate that publishing the data does not compromise anonymity, confidentiality, or breach local data protection laws for the dataset to be considered.
- Consider whether the dataset contains any direct or indirect identifiers and consult your local ethics committee or another appropriate body before submission if there is any possibility that participants will not be fully anonymous.
- State in the manuscript on submission whether informed consent was obtained for publication of patient data. If informed consent was not obtained, state the reason for this and which body was consulted in preparing the dataset.
Software and code guidelines
Authors are required to provide any previously unreported custom computer code or algorithms used to generate the data in their manuscript upon request from editors and reviewers. If the software application or tool is published, it should be freely accessible to any scientist for non-commercial use, without restrictions such as the need for a material transfer agreement. If the implementation is not made available at no cost, the manuscript should concentrate on the development of the underlying method rather than discussing the tool in detail.
A statement detailing how to access the software or custom code must be included in the Declaration section titled “Availability of Data and Materials.” Additionally, license information for the software or method should be clearly stated in this section as well as on the repository site.
This section should also provide a link to the most recent version of your software or code (e.g., GitHub, Sourceforge, or Code Ocean) along with a link to the archived version referenced in the manuscript. The software or code should be stored in an appropriate repository that provides a DOI or other unique identifier. For projects hosted on GitHub, we recommend using Zenodo for archiving.
Any code that has been assigned a DOI must be formally cited and included in the References section of the manuscript.
Any previously unreported software application or custom code described in a manuscript should be available for testing by editors and reviewers while preserving their anonymity.
- The manuscript’s “Availability of Data and Materials” section should describe how editors and reviewers can access the unreported software application or custom code.
- This section should include a link to the most recent version of your software or code (e.g., Zenodo or Code Ocean) and a link to the archived version referenced in the manuscript.
- The software or code should be archived in an appropriate repository with a DOI or other unique identifier. (For GitHub software, we recommend using Zenodo).
- If published, the software application/tool should be readily available to any scientist wishing to use it for non-commercial purposes without restrictions (e.g., no material transfer agreement required).
- If the implementation is not made freely available, the manuscript should focus on the development of the underlying method and not discuss the tool in detail.
Discipline-specific community-recognized repositories
Whenever possible, data should be submitted to discipline-specific, community-recognized repositories. If a suitable discipline-specific repository does not exist, data should be submitted to a generalist repository.
Availability of research materials guidelines
Submitting a manuscript to IJOMAHIP indicates that all materials described in the manuscript, including relevant raw data, will be made freely available to any scientist for non-commercial use. While it is permissible to request a reasonable fee to cover distribution costs, reagents may be provided through either commercial or non-commercial third-party suppliers.
Also, submitting a manuscript to an IJOMAHIP implies that any unique materials described in the manuscript will be freely available to scientists for non-commercial purposes, without breaching participant confidentiality. Research materials include uniquely generated resources such as strains, tools, chemical compounds, antibodies, cell lines, or mutant lines.
- It is acceptable to request reasonable payment to cover distribution costs, and reagents may be made available via commercial or non-commercial third-party providers.
- For biological materials like mutant strains and cell lines, authors are encouraged to use established public repositories where available, and persistent identifiers and/or accession numbers of such resources should be listed in the manuscript.
- Any restrictions on the availability of materials, including if materials are to be distributed by a for-profit company, must be clearly stated in the paper.
For studies where new research materials have been generated, IJOMAHIP encourages including the following statement in the “Availability of data and materials” section:”[REAGENTS/TOOLS/MATERIALS] generated in this study are available from the corresponding author upon request.” Any limitations on the availability of materials, including situations where materials are distributed by a for-profit company, must be clearly outlined in the paper. According to our policy on authorship responsibilities, the corresponding author (or designated authors) is expected to ensure materials availability unless stated otherwise.
Availability of data and materials statements
All authors must include an “Availability of Data and Materials” section in their manuscript to promote transparency and reproducibility, detailing where the data supporting their findings can be found.
- If your data cannot be shared openly, include a statement explaining why. Note that on some journals, editors may decline further consideration if restrictions are unduly prohibitive.
- For clinical trial data, IJOMAHIP encourages data availability statements to follow the ICMJE recommendations on clinical trial data sharing. This includes providing information on:
- Whether individual de-identified participant data (including data dictionaries) will be shared (“undecided” is not an acceptable answer).
- What data will be shared.
- Whether additional, related documents will be available (e.g., study protocol, statistical analysis plan, etc.).
- When the data will become available and for how long.
- By what access criteria data will be shared (including with whom, for what types of analyses, and by what mechanism).
- Example:
- *”Data supporting this study are available in [Repository Name] with DOI [xxxx].”
- “All relevant data are included within the manuscript and supplementary materials.”
Authors must include a Data Availability Statement Encouragement for Repositories: Authors are encouraged to deposit data in trusted repositories like Mendeley Data, Figshare, Zotero, or Zenodo.
Availability of data and materials statements can take various forms, or a combination of forms if needed for multiple datasets.
Data citation policy
IJOMAHIP endorses the Force 11 Data Citation Principles (https://force11.org/info/joint-declaration-of-data-citation-principles-final/) and requires that all publicly available datasets be fully referenced in the reference list with an accession number or unique identifier, such as a digital object identifier (DOI).
Authors must formally cite any datasets referenced in their manuscript, encompassing the primary datasets central to the submission as well as any additional datasets utilized in the research. For datasets that have been previously published, authors should cite both the associated research articles and the datasets themselves. Furthermore, all methods, software, and code developed for the manuscript should be included in the reference list.
A data citation must include the author list and title of the dataset, reflecting the information recorded in the repository. If the repository does not provide an author or title, these should be omitted from the citation. All data citations must include the name of the data-hosting repository, the URL to the dataset, and the year the data became available. For repositories that use DOIs (such as figshare or Dryad), the DOI URL should be cited. For repositories that utilize accession numbers (like SRA or GEO), an identifiers.org URL should be used when available. Please see the following examples for guidance on proper data citation format.
- Citations of datasets in the reference list should include the minimum information recommended by DataCite and follow journal style.
- Dataset identifiers, including DOIs, should be expressed as full URLs.
Example “Availability of data and materials” statement: “The data described in this article can be freely and openly accessed at Harvard Dataverse: https://doi.org/10.7910/DVN/J9EUZU.
Example data citation in the reference list: [1] Correndo AA, Moro Rosso LH, Ciampitti IA. Agrometeorological data using R-software. Harvard Dataverse. 2021. https://doi.org/10.7910/DVN/J9EUZU.
Post-publication data availability policy
IJOMAHIP mandates that all materials described in a manuscript, including all relevant raw data, be freely available post-publication to any scientist wishing to use them for non-commercial purposes without restriction, while protecting participant confidentiality.
- After publication, authors must comply with the data availability statement included in the manuscript and arrange to make the data available to any reader as indicated in the manuscript.
- For datasets with a community norm of sharing data, IJOMAHIP expects that all datasets on which the conclusions of the paper rely are made available to readers via community-accepted repositories linked to the manuscript via permanent identifiers.
- If the original data cannot be produced, the Chief Editor may investigate and reserves the right to contact the institution or funding body.
- Inability to gain access to the requested data for published articles may lead to retraction.
Third party data generation and analysis
When a third party is used to generate or analyze any data presented in the study, this must be clearly stated within the “Methods” and/or “Availability of data and Materials” sections.
- The corresponding author is responsible for all data presented in the published manuscript (refer to the roles and responsibilities of the corresponding author under “Authorship”).
- When data obtained from third parties cannot be made available, the restrictions should be clearly stated in the data availability statement.
- Authors must make data available for purposes of peer review, if requested by reviewers, within the terms of a data use agreement and if compliant with ethical and legal requirements.
Article Processing Charge
All articles published in in IJOMAHIP are open access and available online for free immediately upon publication. This is facilitated by an article-processing charge (APC) that covers a variety of publishing services we offer. These services include providing online tools for Chief Editor, reviewers, and authors, managing article production and hosting, coordinating with abstracting and indexing services, and offering customer support.
The current APC is $100, applicable to accepted manuscripts with up to three authors. For each additional author beyond three, there is a fee of $10, which may be subject to VAT or local taxes where applicable.
The APC is due upon editorial acceptance of your manuscript and must be paid prior to publication. The charge can be billed to you or your funder, institution, or employer.
After acceptance, authors will receive a PayPal invoice for payment. Proof of payment, the final manuscript, and a signed copyright form should be emailed to the editor at ijomahip@virtualrealia.org.
For more information, see Frequently Asked Questions (FAQs) below:
Who is responsible for making or arranging the payment?
As the corresponding author of the manuscript, you are responsible for making or arranging the payment (for example, through your institution) upon the editorial acceptance of the manuscript.
At which stage is the amount I will need to pay fixed?
The article processing charge (APC) that you will need to pay is determined on the date your article is accepted for publication.
When and how do I pay?
Once your article is editorially accepted, you will be notified that payment is due. You must arrange for payment unless a waiver has been granted or if your institution or employer is covering the cost through the Virtualrealia.org Membership Program.
We recommend prompt payment, as we cannot publish accepted articles until payment has been received.
Payment can be made using one of the following methods:
- Credit Card: You can pay online using a secure payment form as soon as your manuscript is editorially accepted. A receipt will be emailed to you once the payment has been processed.
- Invoice: Payment is due within 30 days of editorial acceptance. Receipts are available upon request. Please note that payments made by methods other than credit card may incur an administrative surcharge.

No taxes are included in this charge. If you are a resident of any European Union country, you must add Value-Added Tax (VAT) at the applicable rate for your country. Institutions outside of the UK paying your fee on your behalf can have VAT recorded under the EU reverse charge method, meaning VAT does not need to be added to the invoice; these institutions must provide their VAT registration number. If you reside in Japan, you must add Japanese Consumption Tax (JCT) at the rate set by the Japanese government.
Can charges be waived if I lack funds?
Virtualrealia.org will evaluate requests for waivers from authors experiencing financial difficulties on a case-by-case basis.
Will I have to pay if my institution is a Member?
Many institutions worldwide participate in our Membership Program, which supports open access by covering some or all of the article processing charges (APCs) on behalf of their researchers. If your institution is a member, you will have the option to indicate this after your manuscript is editorially accepted.
I am from a low-income country, do I have to pay an APC?
Virtualrealia.org does not provide APC waivers for manuscripts authored by individuals based in countries classified as low-income economies by the World Bank.
What funding sources are available?
Many funding agencies permit the use of grants to cover article processing charges (APCs). An increasing number of these organizations strongly advocate for open access publication, so please check online for specific details.
APC waivers for substantial critiques of articles published in OA journals
Authors submitting a manuscript that provides a significant critique of an article previously published in the same fully open access journal may apply for a waiver of the APC.
To request an APC waiver on these grounds, please contact IJOMAHIP at the time of submission. Requests will only be considered after a manuscript has been submitted and will be granted at the discretion of the Chief Editor.
What is your APC refund policy?
Virtualrealia.org will issue a refund for an article processing charge (APC) if an error on our part has prevented an article from being published under the open access terms chosen by the authors. This includes situations where an article is not made openly available on the journal platform or is published under a different Creative Commons license than that selected by the authors. A refund will only be provided if these errors have not been corrected within 30 days of publication.
If you notice an error regarding your article’s open access status or licensing, please contact us immediately.
APCs will not be refunded if articles are retracted due to author error or misconduct.
Standards of Reporting
IJOMAHIP promotes complete and transparent reporting in biomedical and biological research. Authors are required to complete the checklist below before peer review and make it available to the Chief Editor and reviewers.
- Randomized controlled trials (CONSORT)
We strongly recommend that authors consult the minimum reporting guidelines for health research provided by the EQUATOR Network when preparing their manuscript, as well as FAIRsharing.org for relevant reporting checklists for biological and biomedical research.
Additionally, IJOMAHIP encourages the use of the following checklists and reporting guidelines:
- Protocols for randomized controlled protocols (SPIRIT)
- Systematic reviews and meta-analyses* (PRISMA) and protocols (PRISMA-P)
- Observational studies (STROBE)
- Case reports (CARE)
- Qualitative research (COREQ)
- Diagnostic/prognostic studies (STARD and TRIPOD)
- Economic evaluations (CHEERS)
- Pre-clinical animal studies (ARRIVE)
- Statistics checklists for editors and reviewers to use when evaluating the statistics in manuscripts:
Please note that IJOMAHIP may have additional reporting guidelines depending on the article type.
Authors of systematic reviews should also provide a link to an additional file from the ‘methods’ section that includes all details of the search strategy. For guidance on presenting a search strategy, see the Cochrane Reviewers’ Handbook.
Statistical methods
Provide comprehensive details on all statistical methods and measures used in your research. Justify the appropriateness of the statistical tests you selected (see SAMPL guidelines). Statistical methods will be reviewed, and specialist statistical review may be required.
Resource identification
To promote tracking of key resources in biomedical literature:
Include a full description of all resources used with sufficient information for unique identification. To support the Resource Identification Initiative (RII), use unique Resource Identifiers (RRIDs) in your manuscript to identify model organisms, antibodies, or tools.
Cell line authentication
If human cell lines are utilized, authors are strongly encouraged to include the following details in their manuscript:
- Source Information: Specify the source of the cell line, including when and where it was obtained.
- Authentication Status: Indicate whether the cell line has been recently authenticated and the method used for authentication.
- Mycoplasma Testing: State whether the cell line has been tested for mycoplasma contamination.
For further guidance, authors can refer to the International Cell Line Authentication Committee (ICLAC). It is also recommended that authors check the NCBI database for any instances of misidentification or contamination of human cell lines.
Gene nomenclature
Standardized gene nomenclature should be used throughout a manuscript. Human gene symbols and names can be found in the HUGO Gene Nomenclature Committee (HGNC) database. Requests for new gene symbols should be submitted to the HGNC, and any inquiries about gene nomenclature can be directed to them. Alternative gene aliases that are commonly used may also be reported, but should not be used alone in place of the HGNC symbol. Nomenclature committees for other species are listed by the HGNC.
The HGNC, a committee of the Human Genome Organization (HUGO), sets the standards for human gene nomenclature, approving a unique and meaningful name for every known human gene based on expert input. In addition to the name, the HGNC also assigns a symbol (a short group of characters) to every gene. The HGNC assigns each named gene a unique symbol, HGNC ID (in the format HGNC:#), and descriptive name. The HGNC database (www.genenames.org) is a curated online repository of HGNC-approved gene nomenclature, gene groups, and associated resources, including links to genomic, proteomic, and phenotypic information. The HGNC has approved almost 43,000 symbols, with around 19,000 for protein-coding genes and the remainder for pseudogenes, non-coding RNAs, and genomic features.
HGNC gene symbol guidelines:
- Gene symbols must be unique.
- Symbols should only contain Latin letters and Arabic numerals.
- Symbols should not contain punctuation.
- Symbols do not contain any reference to the species they are encoded in.
- Symbols should not contain “G” for gene.
The HGNC aims to assign gene names that more accurately describe gene function. Previous symbols will be maintained as synonyms, but the HGNC recommends using the HGNC ID to ensure stability in pipelines and analyses. The HGNC also coordinates with the related Mouse and Rat Genomic Nomenclature Committees, other database curators, and experts for specific gene families or sets of genes.
Reporting of sequence variants
When reporting sequence variants in a manuscript:
- Nomenclature Standards: Follow the recommendations of the Human Variome Project Consortium and the Human Genome Variation Society (HGVS) for describing sequence variants. For describing phenotypes, use the Human Phenotype Ontology. The HGVS nomenclature standard is authorized by the Human Genome Organization (HUGO).
- Database Submission: Submit all variants described in the manuscript to the relevant public gene/disease-specific database (LSDB), and report the database URL and unique identifier in the manuscript.
- HGVS Recommendations: The HGVS nomenclature aims for a consistent and unambiguous description of sequence variants, essential for reporting and exchanging information, especially in DNA diagnostics. The latest recommendations were published in 2016, HGVS version 15.11, focusing on removing inconsistencies and tightening definitions to allow automatic data processing. An extensive version of the recommendations is available online.
- Variant Classification: Instead of using the term “pathogenic”, HGVS recommends using a specific but neutral term like “affects function” to describe the variant’s impact on the gene/protein. Another option is to give both a “functional” and a “clinical” classification for each variant. A five-category functional classification system could use: affects function, probably affects function, unknown, probably does not affect function, does not affect function.
Data
To promote the re-use and utility of published research, authors must adhere to the following:
- Data Standards: Comply with available field-specific standards for data preparation and recording (see the FAIRsharing website).
- Data Sharing: Adhere to best practices for data sharing in your field, prioritizing the maintenance of patient confidentiality.
- Unpublished Genomic Data: If using unpublished genomic data, follow the guidelines of the Fort Lauderdale and Toronto agreements. Contact the owners of unpublished data (principal investigator and sequencing center) before undertaking your research to inform them of your planned analyses.
Digital image integrity
When ensuring digital image integrity in scientific publications:
- Accuracy and Standards: Final images must accurately represent the original data and conform to community standards. Exercise caution during data acquisition to prevent data misrepresentation.
- Transparency: List all image acquisition tools and processing software used, including specific versions. Fully describe and deposit custom code into a community repository if used. Fully describe all image-gathering settings and processing manipulations in the methods section.
- Image Manipulation:
- Do not combine images gathered at different times or locations into a single image unless it is a time-averaged or time-lapse sequence. Clearly demarcate borders and describe them in the legend if juxtaposing images is essential.
- Avoid touch-up tools that deliberately obscure manipulations, such as cloning and healing tools.
- Processing (brightness and contrast adjustments) is only appropriate when applied equally across the entire image and to controls. Avoid contrast adjustments that cause data to disappear.
- Inappropriate manipulations include emphasizing one region at the expense of others or emphasizing experimental data relative to the control.
- Original Data: Be prepared to submit original, unprocessed images upon request.
Maintaining image integrity is crucial for the credibility and authenticity of scientific research. Inappropriate image manipulation has become a significant problem in scientific publishing. Undocumented image manipulations can lead to accusations of research misconduct. Studies suggest that image-related issues are increasing, which could reduce the credibility of scientific literature, lead to costly investigations, and cause reputational damage. AI-powered solutions are emerging to help maintain image integrity in scientific publications. These tools offer automated image proofing, ensuring images adhere to ethical guidelines and are free from unacceptable manipulations, such as duplication, cut-and-paste artifacts, and bands’ deletion in Western blots.
NOTE: Adapted from the Journal of Cell Biology and from Nature Research.
Electrophoretic gels and blots
When presenting electrophoretic gels and blots:
- Cropping: Cropped gels and blots are allowed in the main paper for clarity, but the cropping must be mentioned in the figure legend. The original, uncropped gel or blot is mandatory and should be included in the additional files.
- Quantitative Comparisons: Avoid quantitative comparisons between samples on different gels/blots. If unavoidable, state in the figure legend that the samples are from the same experiment and gels/blots were processed in parallel. Separate vertically sliced images of non-adjacent lanes with a clear separation or black line. Loading controls must be run on the same blot.
- Band Retention: Cropped gels in the paper must retain all important bands.
- Image Quality: Avoid high-contrast gels and blots, as overexposure may mask bands. Aim for exposures with gray backgrounds. If high contrast is unavoidable, provide multiple exposures in the Supplementary Information. Immunoblots with faint backgrounds should have a black line indicating the borders of the blot.
- Quantitation: For quantitative comparisons, use appropriate reagents, controls, and imaging methods with linear signal ranges.
Microscopy
When preparing microscopy images for submission:
- Original Data: Be ready to provide the journal with original microscopy data at the collected resolution upon request.
- Image Integrity: Do not combine cells from different fields into one image. Instead, provide multiple fields as supplementary information. Apply adjustments to the entire image, avoiding threshold manipulation, signal range alterations, and changes to high signals. Disclose the use of pseudo-coloring and nonlinear adjustments (like gamma changes). Note any adjustments to individual color channels in merged images in the figure legend.
- Methods Section: Include the specific equipment used (microscopes/objective lenses, cameras, detectors, filter model and batch number) and acquisition software. List equipment settings for critical measurements, and name any processing software used, indicating manipulations like deconvolution, reconstructions, rendering, gamma changes, filtering, thresholding, and projection.
- Resolution: State the measured resolution at which the image was acquired and any post-acquisition processing or averaging techniques used to enhance the image resolution.
Describing New Taxa Guidelines
Since January 2012, the electronic publication of algal, fungal, botanical, and zoological names has been a valid form of publication. When describing new taxa, different guidelines apply based on the type of organism:
Algal, fungal, and botanical names: Manuscripts that contain new taxon names should adhere to the guidelines set by the International Code of Nomenclature for algae, fungi, and plants. Authors describing new fungal taxa should register the names with a recognized repository, such as Mycobank, and request a unique digital identifier to include in the published article.
Zoological names: Manuscripts containing new taxon names should follow the guidelines set by the International Commission on Zoological Nomenclature. The new taxon name and the article in which it is published should be registered with ZooBank, and the unique identifier provided by ZooBank should be included in the published article.
Bacterial names: In accordance with the International Code of Nomenclature of Prokaryotes (ICNP), effective publication of new prokaryotic names in electronic journals is possible. To comply with the rules of the International Committee on Systematics of Prokaryotes (ICSP) for valid publication, authors must submit a copy of the published article in its final form, along with certificates of deposition of the type strain (for unrestricted distribution), in at least two internationally recognized, publicly accessible culture collections located in different countries, to the International Journal of Systematic and Evolutionary Microbiology (IJSEM) editorial office.
Virus names: The proposal of new virus names must follow the guidelines established by the International Committee on Taxonomy of Viruses (ICTV) in the International Code of Virus Classification and Nomenclature. Proposals for new virus taxa should be forwarded to the relevant Study Group of the ICTV for consideration.
Competing Interests
IJOMAHIP mandates that authors disclose all competing interests related to their work. Each submitted manuscript must include a ‘Competing Interests’ section at the end, detailing all financial and non-financial competing interests.
If authors have no competing interests, the statement should read: “The author(s) declare(s) that they have no competing interests.” The Chief Editor may request additional information regarding competing interests.
Additionally, the Chief Editor and reviewers must also declare any competing interests and may be excluded from the peer review process if such a conflict exists. The statement should read:
- “The authors disclose the following competing interests: [details].”
A completed Declaration of Interests Form must accompany the submission.
What constitutes a competing interest?
Competing interests can be either financial or non-financial. A competing interest arises when the authors’ interpretation of data or presentation of information could be influenced by, or perceived to be influenced by, their personal or financial relationships with individuals or organizations. Authors should disclose any financial competing interests, as well as any non-financial competing interests that could lead to embarrassment if made public after the manuscript’s publication.
Financial competing interests
Financial competing interests encompass (but are not limited to) the following:
- Receiving reimbursements, fees, funding, or salary from an organization that may financially benefit or suffer from the publication of the manuscript, either currently or in the future.
- Holding stocks or shares in an organization that may financially benefit or suffer from the publication of the manuscript, either currently or in the future.
- Holding or actively applying for patents related to the content of the manuscript.
- Receiving reimbursements, fees, funding, or salary from an organization that holds or has applied for patents related to the content of the manuscript.
Non-financial competing interests
Non-financial competing interests encompass a range of factors, including but not limited to political, personal, religious, ideological, academic, and intellectual interests. If you are uncertain about whether you have a competing interest after reviewing these guidelines, please reach out to us for clarification.
Commercial organizations
Authors affiliated with pharmaceutical companies or other commercial organizations that sponsor clinical trials are required to disclose these affiliations as competing interests upon submission. They must also comply with the Good Publication Practice guidelines for pharmaceutical companies (GPP2022), which aim to ensure that publications are created responsibly and ethically. These guidelines extend to any companies or individuals involved in industry-sponsored publications, including freelance writers, contract research organizations, and communications firms. The IJOMAHIP will not publish advertorial content.
Editorial Board Members, Guest Editors and Reviewers
Editorial Board Members, Guest Editors, and Reviewers must declare any competing interests and may be excluded from the peer review process if such interests are identified.
They should also refrain from managing manuscripts in situations where a competing interest exists. This includes, but is not limited to, instances where they have previously collaborated with one or more authors or share the same institution as any of the authors.
If an Editor, Guest Editor, or Reviewer is listed as an author on a manuscript, it is recommended that they disclose this in the “competing interests” section of the submitted manuscript. In cases where they are an author or have another competing interest related to a specific manuscript, another Editor, Guest Editor, or Reviewer will be assigned to oversee the peer review process. These submissions will undergo the same review process as all other manuscripts.
Editorial Board Members are encouraged to submit papers to the journal; however, these submissions will not receive priority over others, and their status as an Editorial Board Member will not influence editorial decisions.
Editorial staff
The editorial staff of IJOMAHIP must disclose any interests—financial or otherwise—that could influence or be perceived to influence their editorial practices to their employer. Failing to do so will result in disciplinary action. IJOMAHIP maintains a strict policy of editorial independence regarding individual manuscript acceptance decisions, ensuring that editorial quality and significance are never compromised. Although some editors may have financial incentives to promote journal growth, our internal policies and individual contracts clearly state that this growth should be achieved by maintaining high submission quality and never at the expense of editorial standards.
Authorship
Authorship acknowledges a researcher’s contributions to a study and entails accountability. Authors are expected to meet the following criteria (adapted from McNutt et al., Proceedings of the National Academy of Sciences, Feb 2018, 201715374; DOI: 10.1073/pnas.1715374115; licensed under CC BY 4.0):
- Each author should have made significant contributions to one or more of the following areas:
- The conception or design of the work
- The acquisition, analysis, or interpretation of data
- The development of new software utilized in the work
- Drafting the manuscript or making substantial revisions
- Authors must approve the submitted version (and any substantially modified version that includes their contributions).
- Authors are required to accept personal accountability for their contributions and ensure that any questions regarding the accuracy or integrity of any part of the work—regardless of their direct involvement—are properly investigated, resolved, and documented in the literature.
IJOMAHIP encourages collaboration with colleagues at research locations and expects their inclusion as co-authors when they meet all authorship criteria outlined above. Contributors who do not fulfill all authorship criteria should be acknowledged in the Acknowledgements section.
For details on how to format author contributions, please refer to the journal’s Submission Guidelines.
Any changes to the author list after submission—including alterations in author order or the addition or removal of authors—must be approved by all authors, and a change of authorship form must be completed. Changes in authorship, including adding or removing authors, altering the Corresponding Author, or changing the sequence of authors, are not permitted after a manuscript has been accepted.
Corresponding authors
Corresponding authors are responsible for ensuring that all listed authors have approved the manuscript prior to submission, including the names and order of authors. They must also ensure that all authors receive the submission, all substantive correspondence with editors, and the full reviews. Additionally, they must verify that all data, figures, materials (including reagents), and code—regardless of whether they were developed or provided by other authors—comply with the transparency and reproducibility standards of both the field and the journal.
This responsibility encompasses several key tasks, including but not limited to:
- Ensuring that original data, figures, materials, and code on which the submission is based are preserved according to best practices in the field for retrievability and reanalysis.
- Confirming that the presentation of data, figures, materials, and code accurately reflects the original.
- Anticipating and minimizing obstacles to sharing the data, materials, and code described in the work.
The corresponding author should manage these requirements across the author group and ensure that all authors are fully aware of and comply with best practices in publication.
To prevent ghost authorship, corresponding authors must disclose whether editorial services were utilized in preparing the manuscript. If such services could represent an undisclosed conflict of interest—such as using an editor from an organization with a vested interest or relying heavily on a technical writer—this should be addressed by including a statement in the acknowledgments, detailing the effort in the methods section, or adding an author.
The contributions of scientific (medical) writers or anyone else who assisted with manuscript preparation should be acknowledged along with their funding sources, in accordance with European Medical Writers Association (EMWA) guidelines. The role of medical writers should be explicitly mentioned in the “Acknowledgements” or “Authors’ Contributions” section as appropriate.
Corresponding authors should also indicate if any authors from earlier versions have been removed or if new authors have been added, along with the reasons for these changes. It is essential for the corresponding author to ensure that all authors (or group/laboratory leaders in large collaborations) have verified the author list and contribution descriptions. This includes confirming that all individuals deserving credit are identified, that no undeserving authors are included, and that any provided author contributions are accurately expressed.
Any potential disputes regarding authorship brought to the editors’ attention will be addressed in accordance with COPE guidelines.
Acknowledgements
All contributors who do not fulfill the criteria for authorship should be included in an “Acknowledgements” section. This may include individuals who provided technical assistance, writing support, or general guidance, such as a Department Chair.
Third party submissions
All manuscripts must be submitted directly by an author and cannot be submitted by a third party.
Author Name Change
Authors who have changed their name due to reasons such as gender transition or religious conversion may request updates to their name, pronouns, and other relevant biographical information on papers published before the change. Authors can opt for this correction to be made discreetly, without any notification of the change appearing on either the PDF or HTML versions of the paper, or they may choose to initiate a formal public Author Correction.
If you are an author who has changed your name and would like to correct it on your published works, please contact us for assistance.
Copyright
When you submit a manuscript, you will need to sign a license agreement allowing IJOMAHIP to publish your work. Here’s what that means for you:
- You, the author, retain the copyright to your article.
- You grant Virtualrealia.org a license to publish your article and identify itself as the original publisher.
- You maintain broad rights to reuse your article content in future publications.
- We publish articles under a Creative Commons license, allowing specific types of reuse by third parties.
- Specifically, articles are published under the CC BY-NC-ND 4.0 International license (Creative Commons Attribution Non-Commercial No Derivatives 4.0 International licence). This means:
CC BY-NC-ND: The article can be shared for non-commercial purposes if you, the authors, are credited. Commercial reuse or sharing altered versions requires permission.
Funder/Institutional Requirements from funders and institutions. See our guide on licensing, copyright, and author rights.
Exceptions: Our policy pages have details about copyright/licensing for articles previously published under different policies (e.g., co-publishing with other publishers). In these cases, access remains free.
For permission and reprint information, please read relevant Editorial Policies.
Artificial Intelligence (AI)
AI authorship
Large Language Models (LLMs), such as ChatGPT, do not currently meet our authorship criteria. Authorship implies accountability for the work, which cannot be effectively assigned to LLMs. Any use of an LLM should be documented in the Methods section of the manuscript, or in an appropriate alternative section if a Methods section is not available. The use of an LLM (or other AI tools) for “AI-assisted copy editing” does not require disclosure.
In this context, “AI-assisted copy editing” refers to AI-driven enhancements to human-generated texts aimed at improving readability and style, as well as correcting errors in grammar, spelling, punctuation, and tone. These enhancements may involve wording and formatting adjustments but exclude generative editorial work and autonomous content creation. Ultimately, there must be human accountability for the final text version, with all authors agreeing that the edits accurately reflect their original work.
AI-generated images and copyright policy
The rapidly evolving field of generative AI image creation has introduced new legal copyright and research integrity challenges. As publisher, we adhere strictly to existing copyright laws and best practices in publication ethics. While legal questions surrounding AI-generated images and videos remain largely unresolved, IJOMAHIP cannot allow their use in publications.
Exceptions:
- Images or artwork obtained from agencies with which we have contractual agreements, provided these images are created legally.
- Images and videos referenced in works specifically discussing AI, which will be evaluated on a case-by-case basis.
- The use of generative AI tools that are based on specific, attributable scientific data that can be verified for accuracy, as long as ethical guidelines, copyright laws, and terms of use are followed.
All exceptions must be clearly labeled as generated by AI within the image field.
Given the anticipated rapid developments in this area, we will regularly review and update this policy as necessary.
Please note that not all AI tools are generative. The use of non-generative machine learning tools to manipulate, combine, or enhance existing images or figures should be disclosed in the relevant caption upon submission for case-by-case evaluation.
Generative AI tools may only be used for language enhancement and must not replace the authors’ intellectual contributions.
- Acceptable Use: Grammar and readability improvements under human supervision.
- Unacceptable Use: Generating or modifying research data, figures, or analyses.
Required statement:
- Include a disclosure in the manuscript, e.g., “The authors used [AI Tool Name] for language refinement. All content was reviewed and validated by the authors.”
AI use by peer reviewers
Peer reviewers are essential to the scientific publishing process. Their expert evaluations and recommendations assist the Chief Editor in making informed decisions, ensuring that published research is valid, rigorous, and credible. Chief Editor selects peer reviewers based on their extensive knowledge of the subject matter or methodologies relevant to the work being evaluated. This expertise is both invaluable and irreplaceable.
Peer reviewers are responsible for the accuracy of their assessments and the opinions expressed in their reports, and the peer review process is founded on mutual trust among authors, reviewers, and Chief Editor. However, despite advancements in technology, generative AI tools have significant limitations: they may not have access to the most current information and can generate nonsensical, biased, or inaccurate content. Additionally, manuscripts may contain sensitive or proprietary information that should remain confidential within the peer review process. Therefore, while IJOMAHIP is exploring ways to provide peer reviewers with access to secure AI tools, we request that reviewers refrain from uploading manuscripts to generative AI tools.
If any aspect of the evaluation of claims made in the manuscript was assisted by an AI tool, we ask peer reviewers to transparently disclose this use in their peer review report.
Citation Guidelines for Research and Non-Research Articles
Articles in IJOMAHIP should be cited in the same way as articles in a traditional journal. Because articles are not printed, they do not have page numbers; instead, they are given a unique article number.
Research and non-research articles (such as Opinion, Review, and Commentary pieces) must appropriately cite relevant literature to support the claims made. Inappropriate practices include excessive self-citation, coordinated self-citation efforts among multiple authors, unnecessary citation of articles published in the journal to which the paper is submitted, and any other form of citation manipulation.
Engaging in citation manipulation will lead to the rejection of the article and may be reported to the authors’ institutions. Likewise, any attempts by peer reviewers or editors to promote such practices should be reported by authors to the publisher.
Authors should consider the following guidelines when preparing their manuscript:
- Any statement that relies on external sources (i.e., not the authors’ own ideas or general knowledge) must include a citation.
- Authors should cite original works instead of relying on derivative sources, such as review articles.
- Citations must be accurate; authors should ensure that each citation supports the statement made in the manuscript and does not misrepresent another work.
- Authors should only cite sources they have personally read.
- Authors should avoid preferentially citing their own publications or those of friends, peers, or their institution.
- Authors should refrain from citing work exclusively from one country.
- Authors should not use an excessive number of citations to support a single point.
- Ideally, authors should reference sources that have undergone peer review whenever possible.
- Authors should not cite advertisements or advertorial content.
Preprint Sharing and Citation
IJOMAHIP encourages authors to share preprints of their primary research manuscripts on preprint servers of their choice, as well as on personal or institutional websites. We support open communication among researchers, whether on community preprint servers or commenting platforms.
Preprints are defined as the author’s version of a research manuscript before it undergoes formal peer review at a journal, deposited on a public server (as described in “Preprints for the Life Sciences,” Science 352, 899–901; 2016). Preprints can be posted at any point during the peer review process and are not considered prior publication, meaning they will not affect consideration at IJOMAHIP. Manuscripts posted on preprint servers will not be factored into the advancement of studies under consideration.
Our policy regarding posting, licensing, citation of preprints, and communication with the media about primary research manuscripts is summarized below:
- Authors should disclose details about their preprint postings, including DOI and licensing terms, when submitting their manuscript or at any time during its consideration at IJOMAHIP. After the preprint is published, it is the author’s responsibility to update the preprint record with a publication reference, including the DOI and a URL link to the published article on the journal’s website.
- Authors may select any license for their preprint, including Creative Commons licenses. The chosen CC license will influence how the preprint can be shared and reused. Additional guidance on licensing options can be found in resource documents developed by an ASAPbio licensing task force.
- Preprints may be cited in the reference list of articles under consideration at IJOMAHIP as illustrated below:
Babichev, S. A., Ries, J., & Lvovsky, A. I. Quantum scissors: teleportation of single-mode optical states by means of a nonlocal single photon. Preprint at http://arxiv.org/abs/quant-ph/0208066 (2002).
- Authors who post preprints are asked to adhere to our policy regarding communications with the media. Researchers may respond to media inquiries related to a preprint or conference presentation by providing explanations or clarifications about their work and its context. Such media coverage will not impede the editorial handling of the submission; however, it may reduce or preempt coverage by other media outlets at publication time. We advise researchers approached by reporters in response to a preprint to clarify that the paper has not yet undergone peer review, that findings are provisional, and that conclusions may change. More information on responsible communication regarding research reported in preprints can be found in resource documents created by the ASAPbio Preprints in the Public Eye project.
For additional information about our self-archiving policies and the release of Author’s Accepted Manuscripts, please contact us.
Duplication Publication
IJOMAHIP upholds strict standards to ensure the originality and integrity of submitted manuscripts. Authors are required to avoid duplication or overlap with other publications and must disclose any potentially overlapping content during submission. Transparency, proper citation, and adherence to ethical guidelines are essential to maintain the quality and credibility of published research. Below is an outline of IJOMAHIP’s policies regarding originality, overlapping publications, and handling of potential misconduct.
Originality and consideration
- All manuscripts submitted to IJOMAHIP must be original. The manuscript, or significant portions of it, should not be under consideration by any other journal. Authors are required to be transparent regarding any potential overlap or duplication.
Declaration of overlapping publications
- Authors must declare any potentially overlapping publications at the time of submission. Any overlapping works should be cited appropriately. If an ‘in press’ or unpublished manuscript is cited or is relevant to the assessment of the manuscript by the Editor and reviewers, it must be made available upon request.
Publication status
- Generally, the manuscript should not have been formally published in any journal or in any other citable format. However, exceptions to this rule may apply if clearly justified at the time of submission. Further details regarding these exceptions can be found below and are summarized in Table 1.
Plagiarism detection and misconduct
- While IJOMAHIP is not currently a member of CrossCheck’s plagiarism detection initiative, it takes publication misconduct seriously. Any suspected cases of covert duplicate manuscript submissions will be addressed according to COPE guidelines.
- All submissions must be original, with a similarity index below 10%.
- The Chief Editor may contact the authors’ institution for further investigation (refer to the Misconduct policy for more information). IJOMAHIP supports the ICMJE policies concerning overlapping publications.
Complete manuscripts
Co-publication agreement
IJOMAHIP currently lacks a co-publication agreement with the Cochrane Library regarding systematic reviews. As such, IJOMAHIP will only consider publishing new Cochrane systematic reviews or updated versions of existing articles from the Cochrane Library if they provide significant new insights.
Co-publication in multiple journals
Co-publication in multiple journals may be permitted at the Chief Editor’s discretion, provided there is transparency and prior agreement from all relevant journals, in accordance with ICMJE guidelines.
Health technology assessment
Reports from the NHS Health Technology Assessment (HTA) program are available online for free. At the Editor’s discretion, some IJOMAHIP journals may consider full or abbreviated versions of these reports for peer review.
Preprint servers and repositories
Posting a manuscript on a preprint server or an author’s personal or institutional website does not count as prior publication. Authors are encouraged to refer to the preprint sharing and citation policy for additional details. IJOMAHIP supports self-archiving for manuscripts accepted for publication.
Theses
Submissions that include material previously part of a PhD or other academic thesis, including publicly available versions as required by the awarding institution, will be considered by IJOMAHIP.
Translations into English
Authors must adhere to ICMJE guidelines when translating works into English. They should obtain approval from the original publisher to ensure compliance with copyright terms and secure permission for publication under the relevant Creative Commons license.
Incomplete manuscripts
Abridged articles
The Chief Editor may accept manuscripts that are significantly expanded versions of previously published articles in peer-reviewed journals. Prior publication of an abridged version does not prevent submission, as long as the new manuscript offers a substantial novel contribution. Authors should seek approval from the original publisher before submitting the extended version.
Abstracts/posters
Previously presented abstracts (up to 400 words) and posters from academic meetings do not disqualify a full manuscript from peer review. The full manuscript is considered a formal advancement in the scientific record, and published abstracts should be cited. Authors should note that many conference proceedings may exceed word limits and can be citable.
Datasets
Making datasets publicly available before submitting associated manuscripts does not hinder consideration by IJOMAHIP. Sharing raw data is encouraged, especially as many funding agencies require it, provided that personal or sensitive information is protected. Refer to the policy on clinical dataset publication for more details.
Non-research articles
- Figures and Tables: Authors of non-research articles (e.g., commissioned reviews and commentaries) may include previously published figures and tables, given that they confirm permission has been obtained from the original publisher and cite the original source. Documentation of this permission must be available upon request.
- Self-plagiarism: To avoid self-plagiarism, authors writing commissioned articles should inform the Chief Editor of any recent publications or similar invitations.
Open science
Authors who have shared their data on blogs, wikis, social media, or electronic lab notebooks can still submit their findings to IJOMAHIP. However, if discussions from these platforms are incorporated into the manuscript, the Chief Editor will evaluate potential duplication.
Study protocols
Publishing study protocols is encouraged as it reduces the risk of non-publication of research findings and promotes methodological discourse. Prior publication of a study protocol does not count as duplicate publication when submitting results.
Summary clinical trial results in public registries
Posting summary results of clinical trials in publicly accessible databases is generally not considered duplicate publication. IJOMAHIP mandates that authors report clinical trials in an appropriate registry (see our Trial Registration policy). In the US, submitting trial results to ClinicalTrials.gov is a legal requirement.
Communication of Findings Prior to Publication
IJOMAHIP supports open communication among researchers and does not consider conference presentations or postings on recognized preprint servers as prior publication. Researchers are encouraged to share their findings through these platforms, including community preprint servers, conferences, wikis, or personal blogs.
Media inquiries related to preprints or conference presentations may be addressed by researchers to provide clarifications or context. Such media coverage will not affect the editorial process. However, researchers should note that early media coverage might reduce subsequent media interest at the time of publication. When engaging with reporters, researchers should emphasize that preprints have not undergone peer review, are provisional, and that conclusions may change. Confidentiality regarding the peer review and editorial processes must also be maintained.
To ensure informed public discussion, IJOMAHIP advises against soliciting media coverage before the peer-reviewed version of a paper is published.
Text Recycling
Authors should recognize that reusing text from their own prior publications is known as text recycling, or self-plagiarism, and may be deemed unacceptable in certain instances. If there is a necessary or unavoidable overlap of text with previous works, it must be disclosed transparently, properly attributed, and compliant with copyright regulations. Authors are required to inform the Chief Editor during submission if their manuscript includes text that has been published elsewhere.
Indexing Services
All articles published in IJOMAHIP are indexed in Scilit and CrossRef, ensuring broad visibility and accessibility within the academic community. This indexing facilitates the dissemination of research findings, allowing them to reach a wider audience and enhancing their discoverability.
By being included in these reputable databases, IJOMAHIP articles benefit from increased exposure, making it easier for researchers and practitioners to locate and utilize the published work.
Peer Review
Peer review is the process utilized to assess the quality of a manuscript before it is published. Independent researchers in the relevant field evaluate submitted manuscripts for originality, validity, and significance, aiding editors in deciding whether the manuscript should be published in the journal.
All research articles, along with most other article types published in IJOMAHIP, undergo a thorough peer review process. Typically, this involves evaluation by two independent reviewers. The peer review procedures may differ among individual journals; for example, some may implement an open peer review system while others use a closed system.
Peer review policy
All submissions to IJOMAHIP are first assessed by the Chief Editor to determine their suitability for peer review. If the Chief Editor is listed as an author or has a conflict of interest regarding a specific manuscript, another member of the Editorial Board will oversee the peer review process. Manuscripts that are deemed suitable for consideration are sent to qualified independent experts chosen by the Handling Editor for review.
Decisions regarding publication are based on the reviewers’ reports, which are provided to the authors along with the editorial decision. Authors should note that even if one reviewer submits a positive report, significant concerns raised by another reviewer can critically affect the validity of the study and may result in the manuscript’s rejection.
Manuscripts submitted to IJOMAHIP are evaluated by our academic and peer reviewers. For manuscripts reporting primary research or secondary analyses of primary research, at least two peer reviews are typically required. However, in exceptional cases—especially in niche or emerging fields—obtaining two independent peer reviewers may not be possible. In such cases, editors may choose to publish based on a single peer review report, provided it meets the required standards. Overall editorial responsibility rests with the Editor, while Editorial Board Members act as handling editors.
IJOMAHIP publishes articles that cater to the needs of specific research communities across all areas of medicine and allied health sciences. Editorial decisions are made based on scientific validity rather than perceived interest or potential impact. Research articles must present a scientifically sound research question, utilize appropriate methods and analyses, and comply with community-agreed standards relevant to the field.
Specific criteria for other types of articles can be found in our submission guidelines. IJOMAHIP is dedicated to being open, inclusive, and reliable in its publishing practices.
AI use by peer reviewers
Peer reviewers are essential to the scientific publishing process, providing expert evaluations that guide editorial decisions and ensure the integrity of published research. They are selected based on their specialized knowledge relevant to the manuscripts they review. This expertise is crucial and cannot be replaced. Peer reviewers are responsible for the accuracy of their assessments, and the peer review process relies on mutual trust among authors, reviewers, and editors.
Despite advancements in technology, generative AI tools have significant limitations, including outdated knowledge and the potential to generate biased or incorrect information. Additionally, manuscripts may contain sensitive or proprietary data that should remain confidential during the review process. Therefore, while IJOMAHIP is exploring the use of safe AI tools for peer reviewers, it is requested that reviewers refrain from uploading manuscripts to generative AI platforms.
If any aspect of a manuscript evaluation is supported by an AI tool, peer reviewers must disclose this usage transparently in their review reports.
Closed peer review
IJOMAHIP employs a closed peer review process, where reviewers remain anonymous and the pre-publication history of each article is not published online.
Peer reviewers
Authors have the option to suggest potential peer reviewers; however, the decision to consider these suggestions lies solely with the Chief Editor. It is important that authors do not recommend recent collaborators or colleagues from their own institution. Suggestions for reviewers can be included in the cover letter, and authors should provide institutional email addresses when possible, or other information that helps the Chief Editor verify the reviewer’s identity (such as an ORCID or Scopus ID).
Additionally, authors may request that certain individuals be excluded from the peer review process, but they must provide reasons for this request in their cover letter upon submission. Authors should be cautious not to exclude too many individuals, as this may impede the peer review process. It is also important to note that the Editor may still choose to invite excluded reviewers.
Deliberately providing false information, such as suggesting reviewers with fictitious names or email addresses, will result in manuscript rejection and may trigger further investigation under our misconduct policy.
Portability of peer review
Within Virtualreali.org
To facilitate an efficient and comprehensive peer review process, we aim to minimize the number of times a manuscript is re-evaluated after being rejected from IJOMAHIP. This approach helps expedite publication and lessens the workload on peer reviewers. If a manuscript does not meet IJOMAHIP’s interest criteria but is sound and relevant to another Virtualreali.org journal, we offer authors the option to transfer the manuscript along with the reviewer reports to that journal.
Before contacting authors, Chief Editor may share manuscripts with Chief Editors of other Virtualreali.org journals to assess their suitability for transfer. Authors who prefer that their manuscript not be shared with other Virtualreali.org journals should indicate this in their cover letter upon submission. Reviewers who wish to keep their reports confidential from other Virtualreali.org journals should express this in the confidential section of their report. It is important to note that transferring a manuscript does not guarantee automatic acceptance by the receiving journal; in some cases, the Chief Editor of the receiving journal may need to conduct their own peer review or reject the manuscript if it is deemed unsuitable.
If a manuscript is transferred to and published in a journal with an open peer review process, we will strive to make the reviewers’ reports available through the article’s pre-publication history whenever possible. However, this may not always be feasible, particularly if the manuscript was initially reviewed by a closed peer review journal. While we will request that reviewers make their reports available, those reviewing for closed peer review journals may choose to maintain confidentiality and anonymity. If a manuscript is first reviewed in an open peer review journal and later transferred to a closed peer review journal for publication, the reviews will not be published alongside the article.
Between IJOMAHIP and other publishers/third parties
IJOMAHIP encourages innovations in the peer review process that enhance efficiency and reduce the burden on peer reviewers. The journal is open to considering manuscripts from other publishers as well as those that have undergone review by third parties. However, submitting a manuscript with reviewer reports from another journal or an independent review service does not guarantee automatic acceptance by IJOMAHIP; additional peer review may still be necessary.
Review process
IJOMAHIP employs a double-blind peer review system to ensure unbiased and thorough evaluation.
- Initial Review:
- Manuscripts are screened by editors for relevance, quality, and ethical compliance.
- Review Process:
- At least two expert reviewers evaluate each manuscript.
- Possible decisions: Accept, Accept with Minor/Major Revisions, or Reject.
- Reviewer Responsibilities:
- Provide constructive and unbiased feedback.
- Maintain confidentiality of the manuscript’s content.
- Disclose any conflicts of interest.
This actual timeframe may vary depending on reviewer availability and the complexity of your manuscript.
Stage 1: Submission and initial review (1-2 days)
- Submit your manuscript following the journal’s guidelines.
- The editorial team will conduct a quick check for completeness (correct format, required files) and suitability for the journal’s scope.
- You will receive notification within 1-2 days informing you if your manuscript is accepted for further review or if it requires revisions before proceeding.
Stage 2: Peer review (1-2 weeks)
- Upon acceptance for full review, your manuscript is assigned to qualified experts in your field.
- Reviewers will evaluate your work based on its originality, relevance, methodology, and overall quality.
- This stage typically takes 1-2 weeks depending on reviewer availability.
Stage 3: Decision and revision (1-2 weeks)
- You’ll receive the editor’s decision (accept, revise with minor changes, or revise and resubmit) along with reviewer comments.
- If revisions are requested, address them carefully and resubmit your manuscript within 1-2 weeks.
Stage 4: Publication (1-2 weeks)
- Once your revised manuscript is accepted, it undergoes copyediting and layout formatting to ensure a professional presentation.
- You’ll be given an opportunity to review the final proofs (galley proofs) for any errors.
- Upon your approval, your research will be published online or in print within 1-2 weeks.
Important Notes
- This expedited process assumes your manuscript is well-prepared, adheres to the journal’s formatting guidelines, and requires minimal revisions.
- Complex manuscripts or those requiring extensive revisions may take longer.
- Consider contacting the journal beforehand to inquire about their typical review timelines for your specific research area.
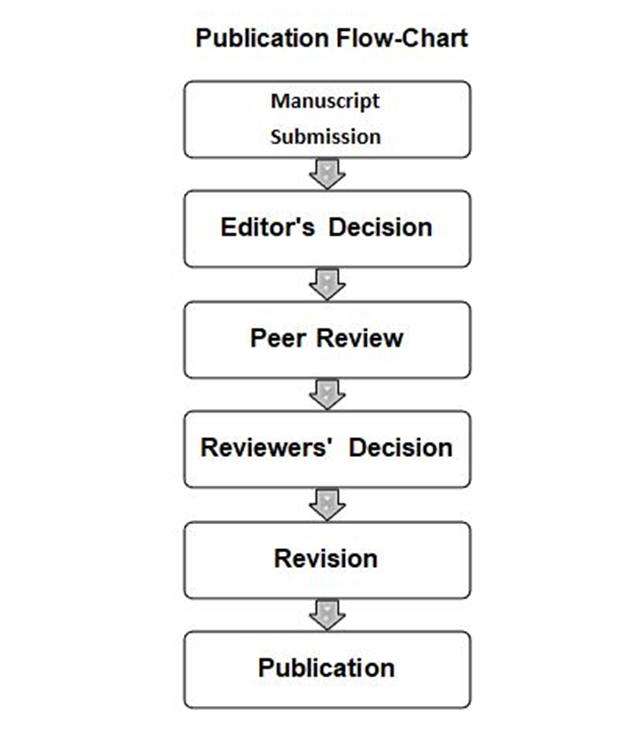
Proofs
Authors will receive proofs of accepted manuscripts for final review before publication.
- Corrections:
- Authors must return proofs within two days.
- Only minor typographical or formatting changes are permitted at this stage.
- Delays:
- Failure to respond within the timeline may delay publication.
Confidentiality
The Chief Editor will maintain confidentiality for all manuscripts submitted to IJOMAHIP. The journal adheres to the COPE’s Ethical Guidelines for Peer Reviewers, requiring reviewers to uphold the confidentiality of the peer review process and refrain from disclosing any details about a manuscript or its review, both during and after the review process, except for information released by the journal. If reviewers wish to involve a colleague in the review, they must first obtain permission from the journal. The names of any individuals who assist in the review must be communicated to the Chief Editor when submitting the review report.
IJOMAHIP will not share manuscripts with third parties outside of Virtualrealia.org unless there are suspicions of misconduct; further details can be found in our Misconduct policy. Manuscripts may be shared with other Editors within Virtualreali.org, unless authors specify otherwise in their submission cover letter. For more information on the portability of peer review, please refer to our guidelines.
Additionally, IJOMAHIP regularly conducts research projects aimed at improving processes for authors, reviewers, and editors, as well as enhancing scientific communication within our journals. Participation in these research initiatives will not influence the editorial review of manuscripts or the consideration of reviewer reports by Editors, nor will it compromise the confidentiality of the submission and review process. Depending on the research project’s nature, we may seek ethical approval and contact authors for consent to participate. Research may also occur retrospectively after manuscript publication; in all cases, manuscript details will be kept confidential.
Potential Research Misconduct
IJOMAHIP takes all allegations of potential misconduct very seriously and adheres to the COPE guidelines for addressing suspected misconduct.
In instances of suspected research or publication misconduct, the Editor may need to contact and share manuscripts with third parties, such as the authors’ institutions and ethics committees. IJOMAHIP may also seek guidance from COPE and discuss anonymized cases in the COPE Forum. A notice regarding any suspected ethical violations in the peer review process may be included in the bibliographic record of the author and article.
Research misconduct
All research involving humans (including human data and materials) and animals must be conducted within an appropriate ethical framework; further details can be found in our Ethics policy. If there is suspicion that research has not adhered to ethical standards, the Chief Editor may reject the manuscript and inform relevant third parties, such as the authors’ institutions and ethics committees.
In cases where research misconduct is proven for published articles or where the scientific integrity of an article is significantly compromised, retraction of the article may occur. For more information, please refer to our Retraction policy.
Data falsification and fabrication
Data falsification involves manipulating research data to create a misleading impression, including altering images, omitting outliers, or modifying data points. Data fabrication refers to inventing research findings.
Any concerns regarding data integrity raised during or after the peer review process will be directed to the Chief Editor. The Editor may request anonymized underlying study data from the authors for verification. If original data cannot be provided, the manuscript may be rejected or, if published, retracted. Cases of suspected misconduct will be reported to the authors’ institutions.
Publication misconduct
IJOMAHIP will follow the COPE guidelines for addressing potential publication misconduct.
Plagiarism
IJOMAHIP uses plagiarism detection software. If plagiarism is identified, we will adhere to COPE guidelines.
Corrections, Retractions and Withdrawal
In rare cases, it may be necessary to publish corrections or retractions for articles in order to uphold the integrity of the academic record.
Corrections or retractions will be issued through a Correction or Retraction notice that is bidirectionally linked to the original article. Any changes made to the original article will be detailed in this notice. The original article will remain publicly accessible, and the subsequent Correction or Retraction will be indexed widely. In exceptional circumstances where material is deemed to infringe on certain rights or is defamatory, we may need to remove that material from our site and associated archive sites.
Authors, readers, or organizations who identify errors or ethical concerns in a published article are encouraged to contact the relevant journal directly using the contact information provided on the journal’s website. All reports will be reviewed by the Chief Editor, who may seek additional expert advice to determine the most appropriate course of action. Virtualrealia.org supports Editors in addressing publication ethics issues in accordance with COPE (Committee on Publication Ethics) guidelines.
Corrections
Errors in published articles that impact their accuracy but do not fundamentally invalidate the conclusions may be corrected at the discretion of the Chief Editor through the publication of a Correction notice. This notice will be indexed and bidirectionally linked to the original article.
Authors who have changed their name and wish to update it on their published works should contact IJOMAHIP for assistance.
Retractions
In rare instances where the interpretation or conclusions of an article are significantly compromised, it may be necessary to retract the published article. The decision to retract is based on the reliability of the article and whether the Chief Editor retains confidence in the interpretations and conclusions presented. In such cases, IJOMAHIP will adhere to COPE guidelines. Retractions serve as a neutral mechanism for correcting the literature and should not be perceived as punitive. Retraction notices will be indexed and linked to the original article, which will be marked as retracted, and its title will be modified to include the prefix “Retracted article:”
Removal of published content
In exceptional cases, IJOMAHIP reserves the right to remove an article, chapter, book, or other content from our online platforms. This action may be taken under the following circumstances:
- if we are informed that the content is defamatory, infringes on a third party’s intellectual property rights, right to privacy, or other legal rights, or is otherwise unlawful;
- (ii) if a court or government order has been issued or is expected to be issued requiring the removal of such content; or
- (iii) if the content poses an immediate and serious risk to health if acted upon.
The removal may be either temporary or permanent. Bibliographic metadata (such as title and authors) will be retained and will include a statement explaining the reason for the content’s removal.
Withdrawal
Authors are permitted to request the withdrawal of their manuscript from consideration at any point before it has been formally accepted. Such requests must be accompanied by a clear and justifiable explanation. Please be aware that withdrawing a manuscript after it has been accepted may result in administrative fees being levied to cover the costs associated with processing the withdrawal.
Editorial Expressions of Concern and Editor’s Notes
Editor’s Note
An Editor’s Note serves as a notification to readers that the journal has initiated an inquiry in response to concerns raised regarding a published article. This update is provided exclusively online and is added only to the HTML version of the published article. It is not indexed.
Editorial Expression of Concern
An Editorial Expression of Concern (EEoC) is a statement issued by the Chief Editor to inform readers of serious issues that may affect the integrity of a published paper. EEoCs are published online, linked bidirectionally to the original paper, assigned a DOI, and indexed in major scholarly databases. They can serve as either an interim measure or a final statement.
The Committee on Publication Ethics (COPE) recommends publishing Chief Editor’s Note or EEoC to keep readers informed during potentially lengthy investigations into research integrity. Typically, but not always, these notices are followed by further amendments—such as a correction or retraction—once the investigation concludes.
Appeals and Complaints
Appeal against a rejection
If you would like the Chief Editor or Editorial Board to reconsider the rejection of your manuscript, please contact the Chief Editor first using the instructions provided on the journal’s website. These requests are classified as appeals and, according to policy, are addressed after the regular workload. Consequently, it may take several weeks to reach a decision on appeals. Only one appeal is allowed per manuscript, and final decisions will be made by the Editorial Board Member overseeing the paper or by the Editor.
Generally, an appeal against a rejection will only be considered if:
- The authors can demonstrate that an error affecting the final decision was made by a referee or the Editor during the review process,
- Important additional data can be provided, or
- A compelling case of bias in the review process can be established.
Authors wishing to appeal an editorial decision should submit a formal letter of appeal to the journal by contacting the editorial office. Please include the manuscript tracking number in both the email subject line and the appeal letter. IJOMAHIP requires an appeal form and will not consider an appeal without it.
If an appeal is successful, authors will receive instructions on how to proceed. Should an appeal warrant further consideration, the Chief Editor may send the authors’ response and revised manuscript out for additional peer review.
For any other feedback or complaints, please refer to the individual journal’s ‘About’ pages for more information.
Complaints
Complaints regarding our processes or publication ethics will initially be addressed by the Chief Editor responsible. If the complaint involves the Chief Editor, please contact the editorial and publishing management team via email at Virtualrealia.org.
- For complaints related to processes, such as the duration of the review, the Chief Editor will assess and respond to the concerns raised by the complainant. This feedback will be shared with relevant stakeholders to help improve processes and procedures.
- For complaints concerning publication ethics or scientific content, the Chief Editor will adhere to the guidelines set forth by the Committee on Publication Ethics. The Editor may seek advice from Virtualrealia.org on complex cases. The Chief Editor will then determine an appropriate course of action and provide feedback to the complainant.
If the complainant remains unsatisfied with how their complaint has been handled, it will be escalated to the journal’s editorial and publishing management team for further investigation.
Collections and Special Issues
All manuscripts submitted to IJOMAHIP Journal Collections or Special Issues are evaluated based on the journal’s standard editorial criteria and are subject to all standard Editorial Policies, including the Competing Interests policy. Additionally, the content of each submission will be reviewed to confirm that it aligns with the scope of the Collection or Special Issue.
Submissions that meet the journal’s criteria for peer review will go through the standard peer review process. For details regarding the review process, please visit the journal’s website. If the Editors of the Collection or Special Issue have any competing interests related to a submission, another Editor without such interests will handle the peer review to ensure an objective evaluation.
ORCiD Identifier
An ORCID iD (Open Researcher and Contributor ID) is a unique digital identifier that distinguishes you and your research outputs, such as articles and datasets. It tackles the problem of name ambiguity in research, ensuring you get credit for your work. You only need to register once, and your ORCID iD remains constant throughout your career, regardless of name changes or institutional affiliations.
Benefits of having an ORCID iD:
- Unique Identification: Provides a unique number for your work on ORCID’s database, helping you gain full credit as an author or contributor1.
- Persistent Association: Persistently connect your name with your research works.
- Discoverability: Helps your research works get discovered within the research community.
- Lifelong Digital Identifier: Your work will always stay under your name and can always be accessed.
- Comprehensive Listing: Create a comprehensive list of your outputs and activities in one place.
- Integration: Virtualrealia.org’s submission systems support ORCID iDs, and you can register for one during the submission process.
- Automatic Profile Filling: If you already have an ORCID iD, the submission system will automatically fill in your profile.
- Enhanced Visibility: The ORCID iD icon will appear next to your name in the article, linking to your ORCID profile.
- Time-Saving: Reduces repetitive data entry.
- Crossref Auto-Update: ORCID records can be automatically updated with your publications.
To register for your free ORCID iD, visit ORCID.org/register
Benefits of Publishing with Us
Publishing with IJOMAHIP offers numerous advantages:
High visibility
The open access policy of IJOMAHIP ensures that articles published in the journal reach a broad, global audience, maximizing their visibility.
Speed of publication
IJOMAHIP provides a rapid publication process while upholding strict peer review standards. All submissions must be made online, and the peer review is conducted entirely electronically, with articles distributed in automatically generated PDF format. Once accepted, articles are published with their final citation in both a fully browsable web format and as a formatted PDF.
Flexibility
Publishing online with IJOMAHIP allows for the inclusion of extensive datasets, numerous color illustrations, and multimedia elements. This format enables data to be presented in a way that can be easily accessed and manipulated by other software, along with the ability to create relevant links.
Promotion and press coverage
Articles in IJOMAHIP are featured in article alerts and regular email updates, with some being highlighted on the journal’s pages or homepage.
Additionally, selected articles may be promoted through press releases to both general and scientific media, enhancing their exposure and accessibility. A list of recently press-released articles from IJOMAHIP is available for reference
Copyright
As an author publishing in IJOMAHIP, you retain full copyright ownership of your article but grant IJOMAHIP the right of first publication. Articles are published under a Creative Commons Attribution (CC BY) license, allowing authors to share their work freely. Authors may also enter into separate non-exclusive agreements for its distribution, such as uploading to repositories. IJOMAHIP supports open-access practices, encouraging authors to post their work online before and during the submission process.
- Creative Commons Options:
- CC BY: Unrestricted use with proper attribution.
- CC BY-NC: Non-commercial use with attribution.
- CC BY-NC-ND: Non-commercial use without derivatives.
- Author Rights:
- Authors retain copyright while granting IJOMAHIP the right of first publication.
You are free to reproduce and share your work without restrictions. For detailed guidance on licensing and author rights, consult the journal’s dedicated resources on these topics.
On Archiving and Preservation
Long-term preservation of published content is a priority for this journal. We are developing archiving solutions to ensure that content remains accessible. In the meantime, we maintain regular backups to safeguard against data loss..
Future Plans
The journal is committed to secure and permanent archiving through the implementation of trusted systems, such as CLOCKSS or LOCKSS.
Current Approach
Published articles remain accessible on the journal’s official website.
To explore more advantages of publishing with IJOMAHIP, visit the journal’s comprehensive information section.
